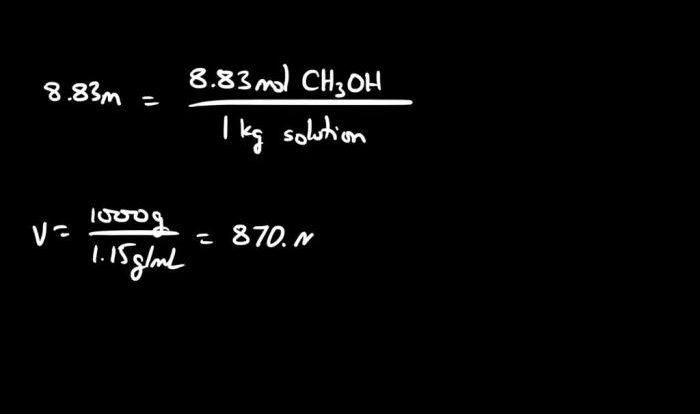
Welcome to our in-depth exploration of the limiting reagent and percent yield worksheet answer key, an essential resource for understanding the intricacies of chemical reactions and their outcomes. This guide will provide a comprehensive overview of the concepts, calculations, and applications of limiting reagents and percent yield, empowering you with the knowledge to excel in your studies and deepen your understanding of chemistry.
Limiting Reagent and Percent Yield Worksheet Answer Key

This worksheet answer key provides step-by-step solutions to problems involving limiting reagents and percent yield. Understanding these concepts is crucial for predicting the outcome of chemical reactions and optimizing their efficiency.
Limiting Reagent
The limiting reagent is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. To identify the limiting reagent:
- Calculate the mole ratio of each reactant to the stoichiometric coefficients in the balanced chemical equation.
- The reactant with the smallest mole ratio is the limiting reagent.
Example:
Consider the reaction: 2H 2+ O 2→ 2H 2O
If we have 4 moles of H 2and 2 moles of O 2:
- Mole ratio of H 2: 4 moles / 2 = 2
- Mole ratio of O 2: 2 moles / 1 = 2
Since both reactants have the same mole ratio, neither is limiting. However, if we have 4 moles of H 2and 1 mole of O 2:
- Mole ratio of H 2: 4 moles / 2 = 2
- Mole ratio of O 2: 1 mole / 1 = 1
In this case, O 2is the limiting reagent because it has the smallest mole ratio.
Percent Yield, Limiting reagent and percent yield worksheet answer key
Percent yield is a measure of the efficiency of a chemical reaction, calculated as the ratio of the actual yield (amount of product obtained) to the theoretical yield (amount of product predicted by stoichiometry), multiplied by 100%.
Formula:
Percent yield = (Actual yield / Theoretical yield) × 100%
Factors that can affect percent yield include:
- Stoichiometric imbalances
- Side reactions
- Incomplete reactions
- Losses during purification
Example:
If a reaction with a theoretical yield of 100 grams produces 80 grams of product, the percent yield is:
Percent yield = (80 grams / 100 grams) × 100% = 80%
Worksheet Answer Key
| Problem | Answer |
|---|---|
| Calculate the limiting reagent in the reaction: 2Al + 3Cl2 → 2AlCl3, given 4 moles of Al and 2 moles of Cl2. | Cl2 |
| Calculate the percent yield of a reaction that produces 12 grams of product when the theoretical yield is 15 grams. | 80% |
Practice Problems
- Identify the limiting reagent in the reaction: 4Fe + 3O 2→ 2Fe 2O 3, given 10 moles of Fe and 5 moles of O 2.
- Calculate the percent yield of a reaction that produces 10 grams of product when the theoretical yield is 12 grams.
- Explain how the presence of impurities can affect the percent yield of a reaction.
Questions and Answers: Limiting Reagent And Percent Yield Worksheet Answer Key
What is a limiting reagent?
A limiting reagent is a reactant that is completely consumed in a chemical reaction, determining the maximum amount of product that can be formed.
How is percent yield calculated?
Percent yield is calculated by dividing the actual yield of a reaction by the theoretical yield and multiplying by 100.
What factors can affect percent yield?
Factors that can affect percent yield include side reactions, incomplete reactions, and loss of reactants or products.