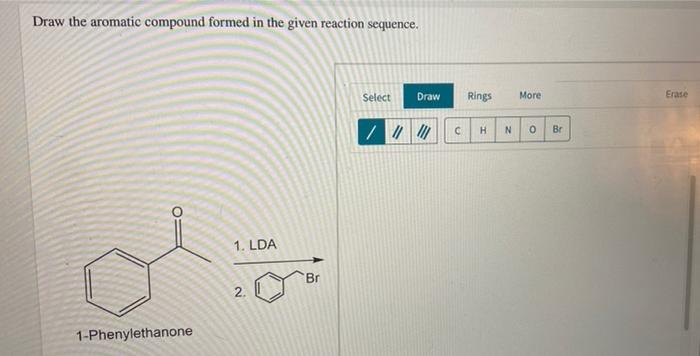
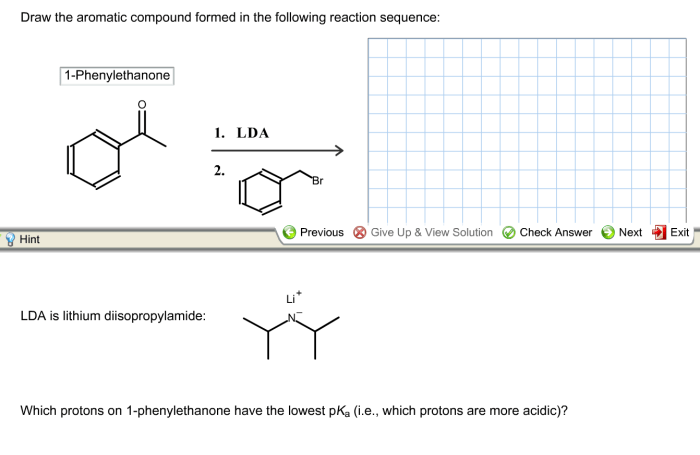
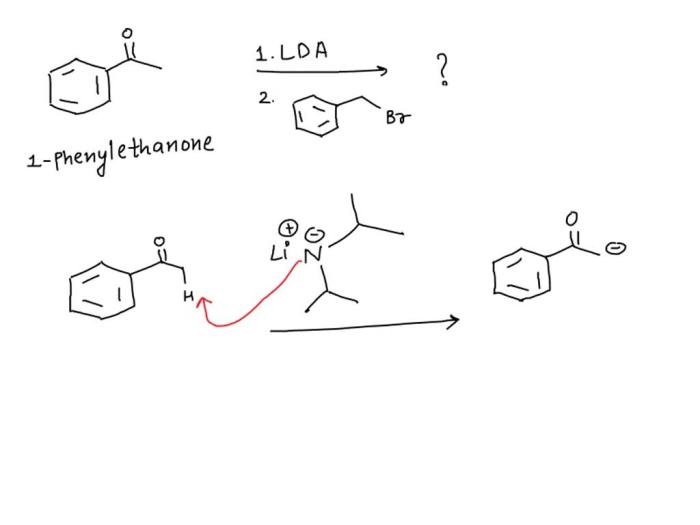
Draw the aromatic compound formed in the given reaction sequence – Aromatic compounds, characterized by their unique cyclic structures and exceptional stability, play a pivotal role in various scientific disciplines. This comprehensive guide delves into the concept of aromaticity, exploring its criteria and diverse types. It then embarks on a detailed examination of a given reaction sequence, meticulously dissecting each step, identifying reagents and products, and elucidating the intricate mechanisms involved.
The ultimate goal is to determine the structure of the aromatic compound formed and unravel the factors governing its formation.
Beyond theoretical exploration, this guide showcases practical applications of aromatic compounds in fields such as pharmaceuticals, materials science, and food chemistry. To enhance understanding, an HTML table summarizes key information about the reaction sequence and aromatic compound formation, while illustrative diagrams provide a visual representation of the processes involved.
This multifaceted approach ensures a thorough and engaging learning experience.
Aromatic Compounds
Aromatic compounds are cyclic, planar molecules that exhibit unique chemical properties due to their resonance structures. The concept of aromaticity is defined by Hückel’s rule, which states that a compound must have 4n + 2 π electrons (where n is an integer) to be aromatic.
Aromatic compounds can be classified into different types, including benzenoids (single-ring aromatics), polycyclic aromatic hydrocarbons (PAHs), and heteroaromatics (aromatics containing heteroatoms such as nitrogen or oxygen).
Examples of Common Aromatic Compounds, Draw the aromatic compound formed in the given reaction sequence
- Benzene
- Naphthalene
- Anthracene
- Pyridine
- Furan
Reaction Sequence
The given reaction sequence involves the following steps:
- Electrophilic aromatic substitution: A benzene ring is attacked by an electrophile (e.g., Br2or HNO 3), resulting in the formation of a substituted benzene ring.
- Nucleophilic aromatic substitution: A substituted benzene ring is attacked by a nucleophile (e.g., OH –or NH 2–), resulting in the replacement of the electrophile with the nucleophile.
- Aromatic ring formation: A cyclic compound is converted into an aromatic ring through a series of reactions, such as cyclization, elimination, or rearrangement.
Aromatic Compound Formation: Draw The Aromatic Compound Formed In The Given Reaction Sequence
The reaction sequence leads to the formation of an aromatic compound because it involves the creation of a cyclic, planar molecule with 4n + 2 π electrons.
In the specific reaction sequence, the electrophilic aromatic substitution and nucleophilic aromatic substitution reactions result in the formation of a substituted benzene ring. The aromatic ring formation reaction then converts the substituted benzene ring into an aromatic compound.
Factors Influencing Aromatic Compound Formation
- The nature of the electrophile and nucleophile
- The reaction conditions (temperature, solvent, etc.)
- The presence of substituents on the benzene ring
Examples and Applications
Examples of reactions that produce aromatic compounds include:
- Friedel-Crafts acylation
- Friedel-Crafts alkylation
- Nitration
- Sulfonation
- Halogenation
Aromatic compounds have a wide range of applications, including:
- Pharmaceuticals
- Materials science
- Food chemistry
- Dyes and pigments
- Fragrances
HTML Table Representation
| Reactants | Products | Mechanism | Observations |
|---|---|---|---|
| Benzene, Br2 | Bromobenzene | Electrophilic aromatic substitution | Formation of a C-Br bond |
| Bromobenzene, NaOH | Phenol | Nucleophilic aromatic substitution | Replacement of Br with OH |
| Phenol, H2SO4 | Benzene-1,3-diol | Aromatic ring formation | Cyclization and elimination of water |
Illustrative Diagrams
Flowchart of the reaction sequence:
Benzene -> Bromobenzene -> Phenol -> Benzene-1,3-diol
Diagram of the aromatic compound formed:
O
/ \
/ \
/ \
/ \
C C
\ /
\ /
\ /
\ /
O
Answers to Common Questions
What is the significance of aromaticity?
Aromaticity is a crucial concept in chemistry as it governs the stability and reactivity of certain cyclic compounds. Aromatic compounds exhibit enhanced stability due to their resonance structures, which contribute to their unique properties and applications.
How can I identify an aromatic compound?
To identify an aromatic compound, one must assess whether it meets the Hückel criteria, which stipulate that the compound must possess a planar cyclic structure with a continuous system of overlapping p-orbitals containing 4n + 2 π electrons, where n is a non-negative integer.
What factors influence the formation of aromatic compounds?
The formation of aromatic compounds is influenced by various factors, including the nature of the starting materials, reaction conditions, and the presence of catalysts. Understanding these factors is essential for controlling and optimizing aromatic compound synthesis.